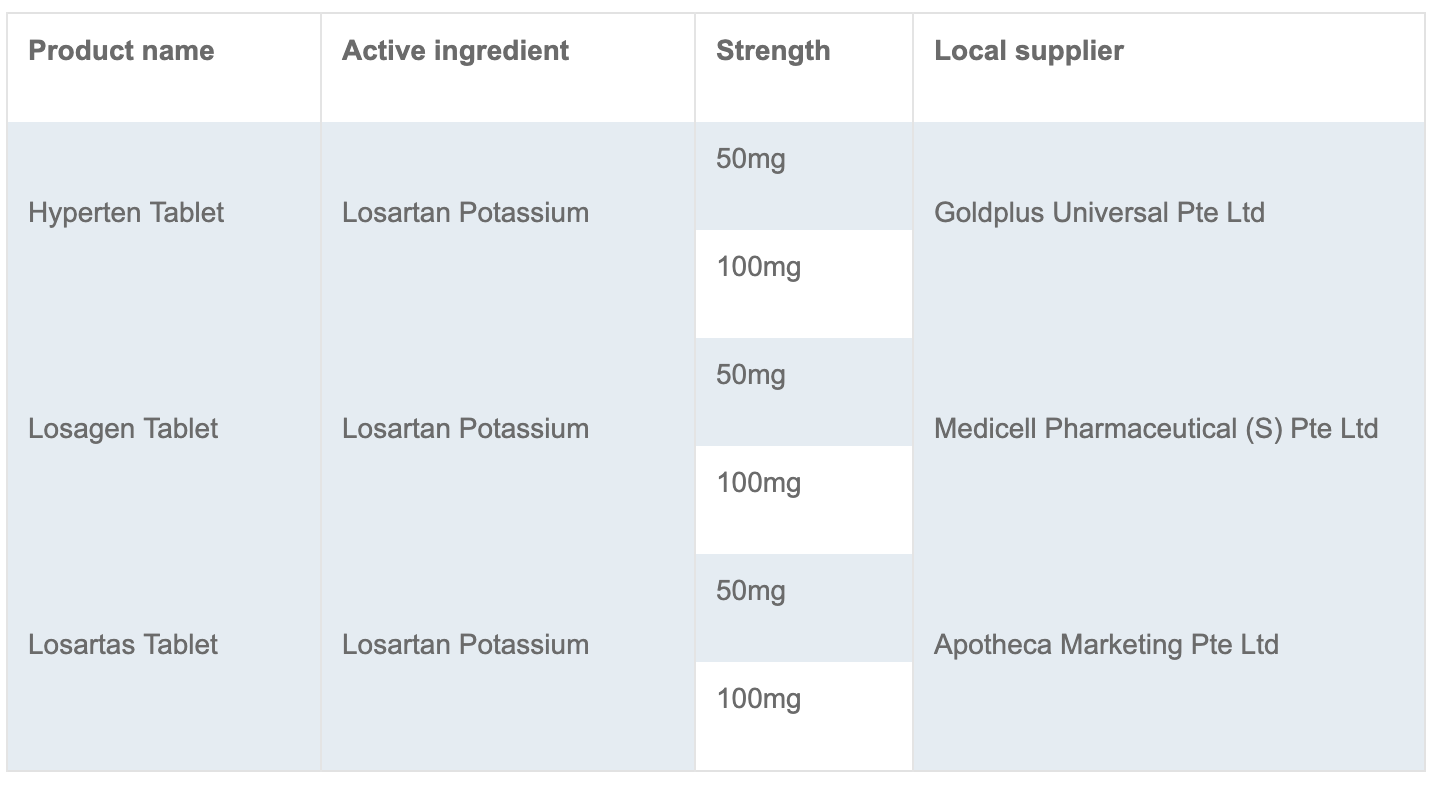
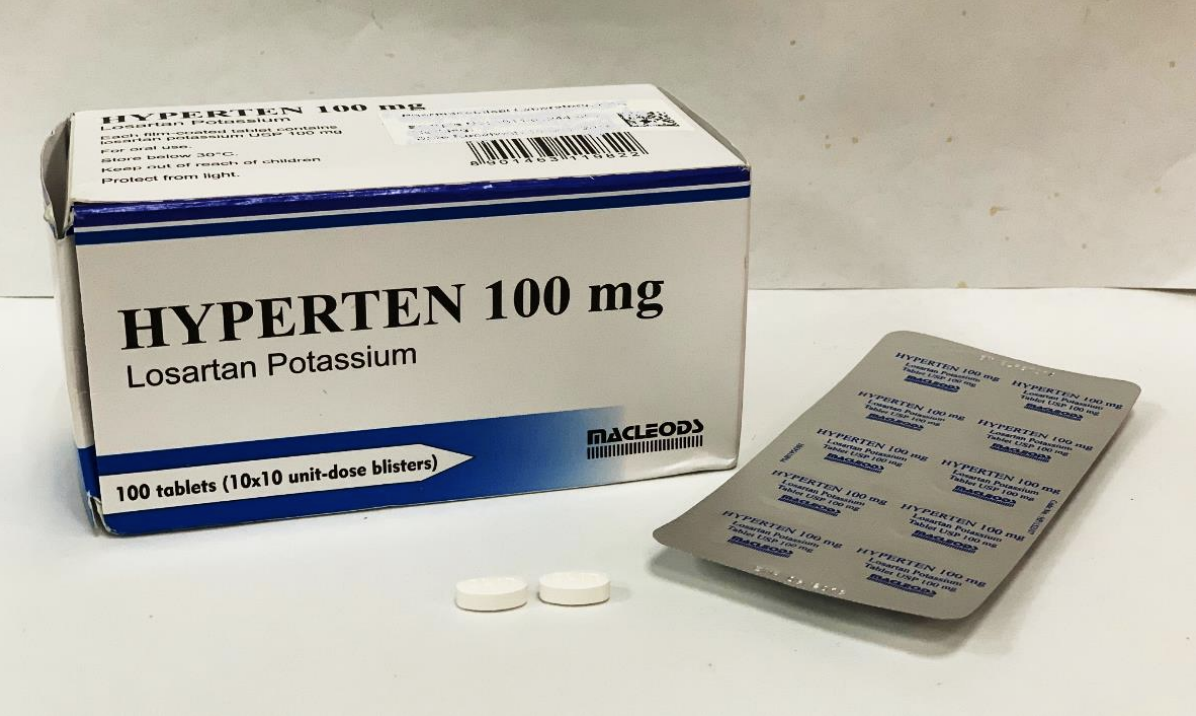
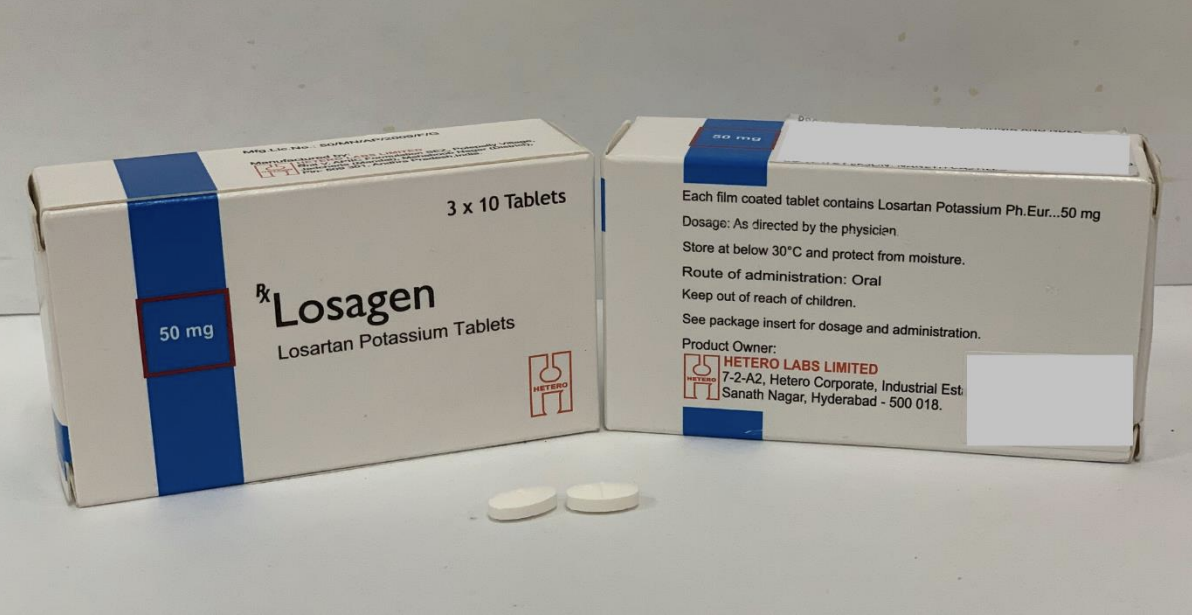
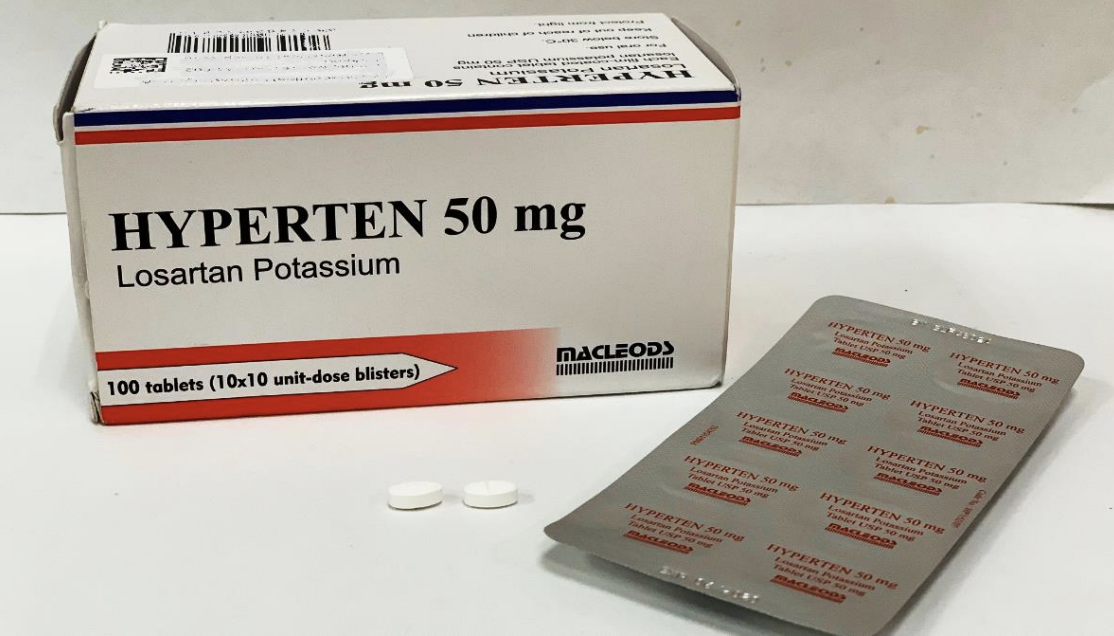
The Health Sciences Authority (HSA) is recalling three brands of blood pressure medicines which contain a losartan ingredient manufactured by Hetero Labs.
These products were found to contain trace amounts of a nitrosamine impurity, which are above internationally acceptable levels.
HSA urged patients not to stop treatment on their own as there is no immediate health risk associated with taking the affected medicines. Healthcare professionals have been advised review the medicine and treatment plans of their patients.
Meanwhile, the authorities are working with companies and international regulatory agencies to verify the cause of contamination, and to formulate measures to address the issue.
"There is no immediate health risk associated with taking the affected medicines, and patients are advised not to stop or change treatment on their own," said Professor Ding Zee Pin, Cardiologist in the National Heart Centre Singapore and HSA’s Expert Panel on Nitrosamines.
Stopping the medicine without replacement of other equivalent medication can increase the risk of poor control of blood pressure, he added.
Consumers should consult their healthcare provider if you are unsure if you are taking an affected brand, HSA said.
Affected products: